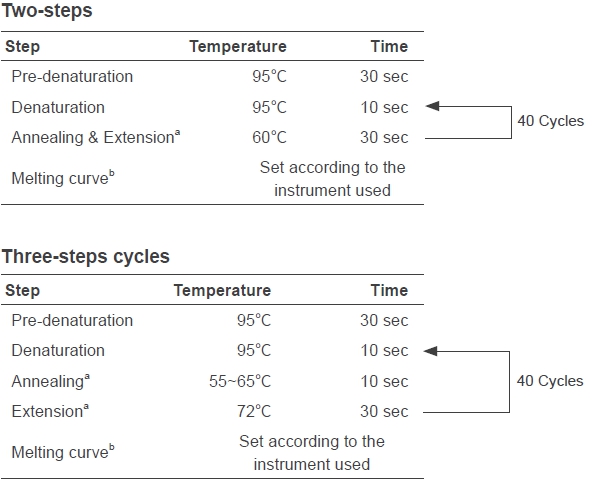
Taq SYBR® Green qPCR Premix
REF : 201001

Storage Condition
Components

Description
Taq SYBR® Green qPCR Premix is a special premix for qPCR
detection using SYBR Green I chimeric fluorescence method.
The Mix contains all components for qPCR, except DNA templates
and primers. It reduces the reaction set-up time and risk of
contaminations. The core component is the antibody modified hot-start
Taq DNA polymerase. With the optimized buffer and PCR enhancer,
the Mix has high efficiency and specificity that suppresses non-specific
amplifications well to obtain consistent and reliable qPCR results in a
broad template concentration range.
This product contains universial correction dye that are compatible
with all of qPCR devices, including instruments that require ROX
calibration, and do not require additional dyes to correct the instrument.
Method of Application
1. Notes
① Avoid strong light exposure during usage and storage to protect the pre-mixed dye
② Mix gentle and thoroughly by turning the tube up and down before use. Do not vortex to generate excessive bubbles;
③ This mixture contains universal corrective dyes for all qPCR instrucments.
2. Prepare the following mixture in a qPCR tube
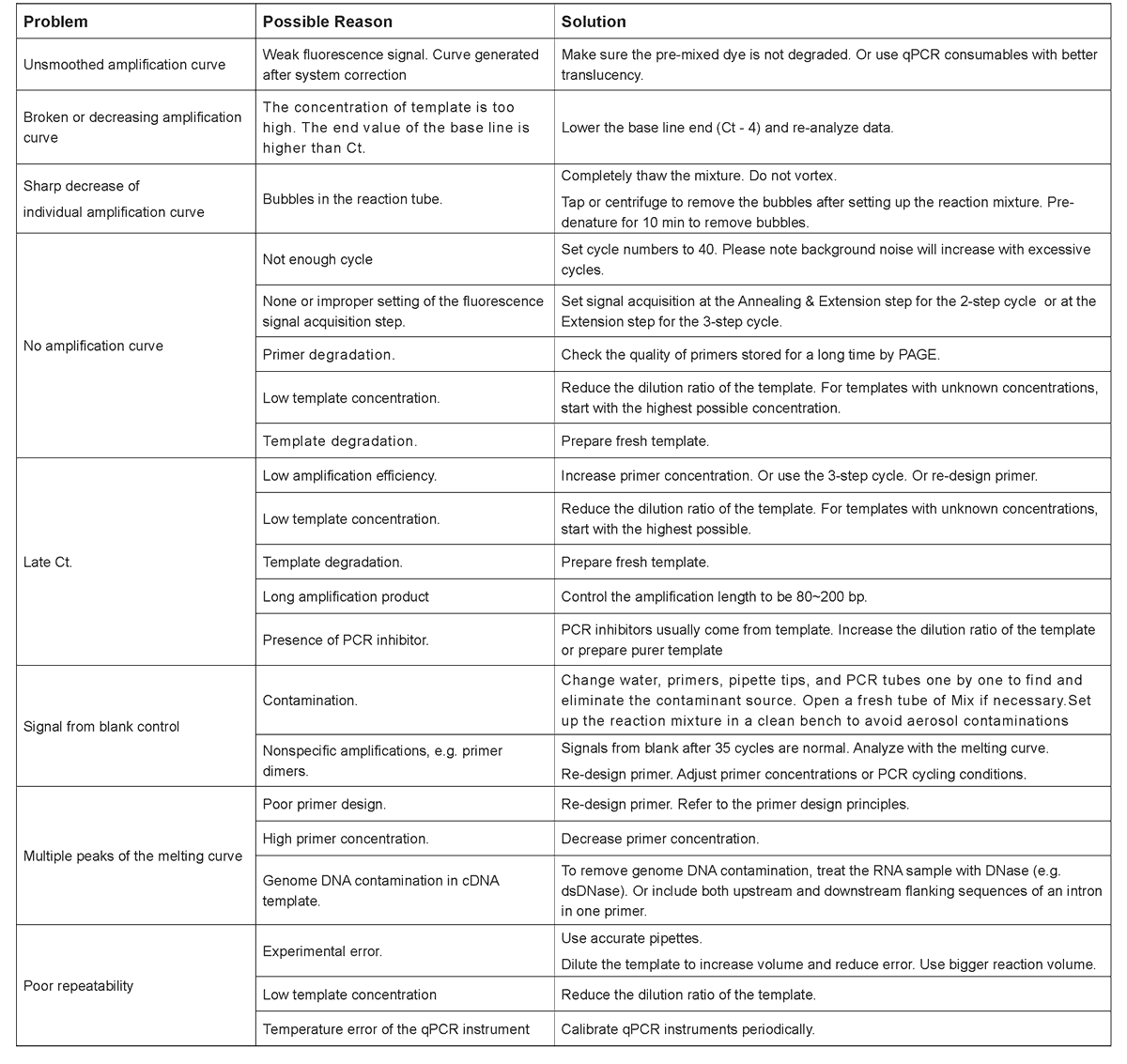
a. Commonly used primer concentration: 0.2 μM final. Adjust between 0.1~1 μM;
b. Suggested template volume: 1~2 μl. Do not exceed 10% of the total volume if the template is undiluted cDNA. As the copy number of target gene in different DNA templates varies, gradient dilution can be performed if necessary to determine the optimal amount of DNA template.
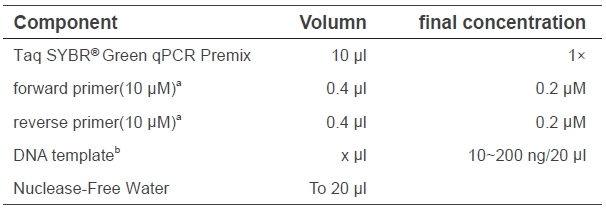
3. qPCR program(Adjust according to the instrument used)
a. Set Annealing & Extension or Annealing temperatures according to the Tm of primers. Set Annealing & Extension or Extension time at 15 sec for < 200bp amplifications. Please also consider the minimum data acquisition time of the instrument used for the time setting;
b. Default programs of instrument are usually used.
4.Optimizations
When ideal results are not obtained using the default conditions,optimizations are necessary:
① Primer concentration adjustment
When final concentration of primers is in the range of 0.1~1.0 μM, the lower concentration leads to the higher the amplification specificity, but the amplification efficiency will decrease.② Primer design principles
If higher amplification specificity is desired, use the two-steps cycle or increase the annealing temperature. If higher amplification efficiency is desired, use the 3-step cycle or increase the extension time.5.Primer design principles
② Primer length: 18~25 bp;
③ Difference of the Tm between two primers should less than 1 ℃ . Suggested Tm range: 58~62℃ ;
④ Primer GC content: 40%~60%;
⑤ Keep an uniform distribution of A, G, C, and T in the primers; Avoid repeated T/C or A/G (especially near the 3' end);
⑥ The 3' end of a primer is better to be a C or a G;
⑦ Avoid complementary sequences in a primer or between two primers;
⑧ Confirm the specificity of primer with NCBI primer-BLAST.
FAQ & Troubleshooting